
Related Posts

Today’s release of new 2020 Census data provides population counts of nearly 1,500 race and ethnicity groups and American Indian and Alaska Native (AIAN) tribes and villages.

NABP Associate Executive Director Josh Bolin on DSCSA compliance prior to FDA moving compliance deadline.
Using Public Health Detailing to Increase Access and Confidence in COVID-19 Vaccines and Reinvest in Disproportionately Impacted NYC Communities Go…

‘Unbelievably good price’: KC-area pharmacy dispenses bargain medicine — and hope Go to article

APPLICATIONS ARE OPEN! – Letters of Intent due 9/15/23
Uninsured adults and those in worse health continue to report higher rates of not getting care due to costs
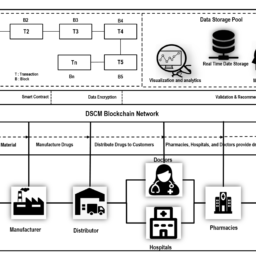
FDA approves 1 Year delay of Track & Trace requirements
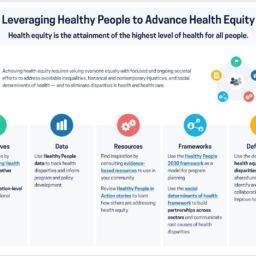
Healthy People 2030 Leveraging Healthy People to Advance Health Equity Health equity is the attainment of the highest level of health for all people. “Eliminate health disparities, achieve health equity, and attain health literacy to improve the health and well-being of all.”

Branson MO: My Neighbor’s Charitable Pharmacy (NCP) will open its doors at 1232 Branson Hills Parkway February of 2023
The pharmacy industry sees at least five changes
Significant improvement in glycemic control among participants demonstrates the substantial impact that pharmacies partnered with charitable medication distributors such as the Dispensary of Hope can have on individuals with insulin-treated T2D

How is a charitable pharmacy different from other types of pharmacies?

St. Vincent de Paul Charitable Pharmacy offers article regarding impact of a charitable pharmacy on their community and patients
We estimate that if the PHE expires in April 2023, 18.0 million people will lose Medicaid coverage in the following…

Three Healthcare Organizations Join Forces to Save Lives

