Three Healthcare Organizations Join Forces to Save Lives

NABP Associate Executive Director Josh Bolin on DSCSA compliance prior to FDA moving compliance deadline.

Today’s release of new 2020 Census data provides population counts of nearly 1,500 race and ethnicity groups and American Indian and Alaska Native (AIAN) tribes and villages.

Just as banks have helped seniors bridge the digital divide and leverage online banking to better manage their money, healthcare organizations need to help older adults leverage digital technology to better manage their health.

St. Vincent de Paul Charitable Pharmacy offers article regarding impact of a charitable pharmacy on their community and patients
How a ‘weighted lottery’ helped underserved patients get a scarce Covid drug
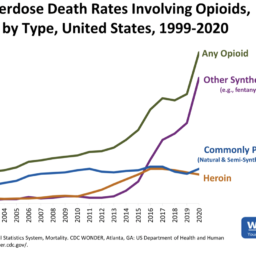
Patients taking opioid dosages at or above 50 MME/day are twice as likely to overdose compared to those taking 20 MME/day, and the risk further increases as the MME/day increases.

The five most recent states to expand the scope of reimbursement for pharmacists are Maryland, Missouri, North Dakota, Virginia and Wyoming
Significant improvement in glycemic control among participants demonstrates the substantial impact that pharmacies partnered with charitable medication distributors such as the Dispensary of Hope can have on individuals with insulin-treated T2D

Recent federal legislative and regulatory updates in managed care pharmacy have prioritized topics ranging from expedited access to novel therapeutics to the health disparities and equity concerns affecting patient populations nationwide, but progress on these developments will depend on the impact of the midterm elections
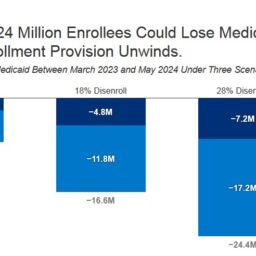
How Many People Might Lose Medicaid When States Unwind Continuous Enrollment?

Medication non-adherence has led to at least 100,000 preventable deaths each year and $100 billion annually in preventable healthcare costs in the United States
We estimate that if the PHE expires in April 2023, 18.0 million people will lose Medicaid coverage in the following…

APPLICATIONS ARE OPEN! – Letters of Intent due 9/15/23
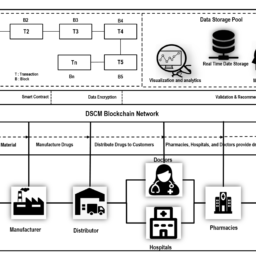
FDA approves 1 Year delay of Track & Trace requirements

