Related Posts

Charitable Pharmacies of America organization member receives Health Equity grant

Charitable Pharmacies of America organization member receives Health Equity grant

One in four Americans say they struggle to afford their prescription medications. The establishment of this Charitable Pharmacy in Milford will allow patients who live east of Cincinnati better access to care.

This toolkit, comprised of fact sheets, worksheets, sample communications materials, and sample social media is designed to help aid how you support your own mental health journey, along with your community’s.
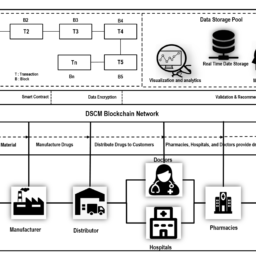
FDA approves 1 Year delay of Track & Trace requirements

The five most recent states to expand the scope of reimbursement for pharmacists are Maryland, Missouri, North Dakota, Virginia and Wyoming
We estimate that if the PHE expires in April 2023, 18.0 million people will lose Medicaid coverage in the following…

St. Vincent de Paul Charitable Pharmacy offers article regarding impact of a charitable pharmacy on their community and patients
Uninsured adults and those in worse health continue to report higher rates of not getting care due to costs

‘Unbelievably good price’: KC-area pharmacy dispenses bargain medicine — and hope Go to article

The Outcomes of Implementing and Integrating Comprehensive Medication Management in Team-Based Care: A Review of the Evidence on Quality, Access and Costs, December 2023

There are multiple factors that will impact an individual patient’s choice on how to go about getting their prescription medication. It can be overwhelming to navigate this complex issue.

Federal/state grants are not the only gateways to funding. There are close to 750 “community foundations” nationwide which are public charities dedicated to improving the lives of people in a defined local geographic area.

An opportunity for charitable pharmacies to collaborate with oncology practices for non-oncology medications during and after treatment.

Good Pill will provide direct access to hundreds of life-saving medications and save families $150+/month on healthcare cost

