Related Posts

Charitable Pharmacies of America organization member receives Health Equity grant

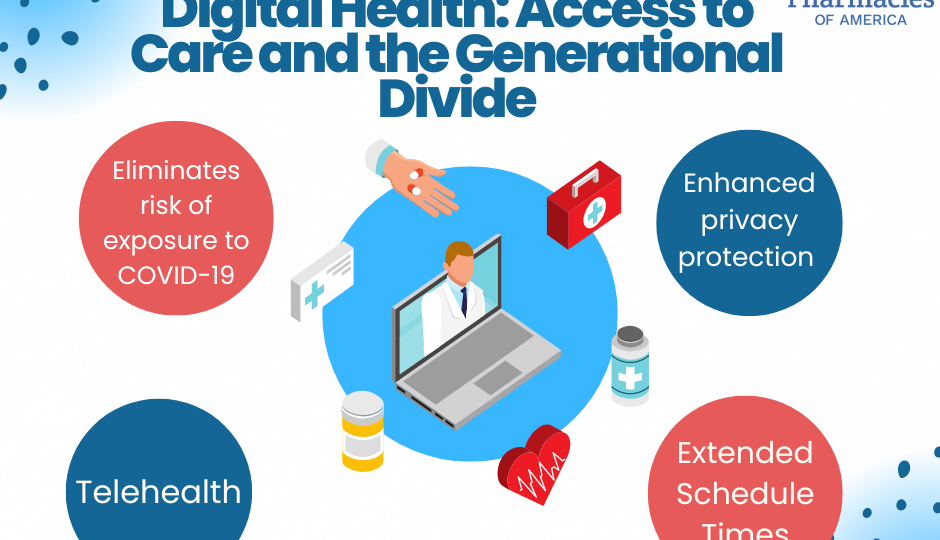
Implications for Patients
Once the continuous enrollment ends, it is estimated between 5 -14 million individuals will be affected

‘Unbelievably good price’: KC-area pharmacy dispenses bargain medicine — and hope Go to article
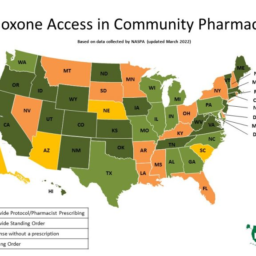
In 2011, the opioid crisis reached astounding levels with over 21,000 deaths adding to the urgency of providing aid to those who most need it

An opportunity for charitable pharmacies to collaborate with oncology practices for non-oncology medications during and after treatment.

How is a charitable pharmacy different from other types of pharmacies?

NOVA ScriptsCentral is excited to collaborate with the American Pharmacist Association to launch the “NOVA ScriptsCentral & American Pharmacist Association Safety-Net Health Equity Fellowship”.

This study represents a rigorous, multi-state evaluation that highlights the impact of a charitable medication access program on hospital utilization for the medically under-served population.

Charitable Pharmacies of America organization member receives Health Equity grant

Federal/state grants are not the only gateways to funding. There are close to 750 “community foundations” nationwide which are public charities dedicated to improving the lives of people in a defined local geographic area.
Using Public Health Detailing to Increase Access and Confidence in COVID-19 Vaccines and Reinvest in Disproportionately Impacted NYC Communities Go…
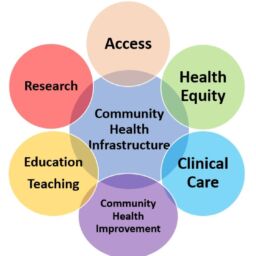
An opportunity for charitable pharmacies to collaborate with oncology practices for non-oncology medications during and after treatment.

The five most recent states to expand the scope of reimbursement for pharmacists are Maryland, Missouri, North Dakota, Virginia and Wyoming
Healthy People 2030 Leveraging Healthy People to Advance Health Equity Health equity is the attainment of the highest level of health for all people. “Eliminate health disparities, achieve health equity, and attain health literacy to improve the health and well-being of all.”


